The COVID-19 Back-to-normal study was initiated in January 2021 as an effort of a tight-knit neighborhood to help each other avoid the virus and vaccinate safely.
Later the research protocol was approved by MEBO Research IRB and the study was open to other communities around the world.
By now, we have over 600 participants.
Early results of the study in MEBO/PATM community, based on the replies of the first 26 enrollees, showed that while reactions to vaccine were similar to the general population, experiences with COVID-19 infections were not - 2 individuals were not able to avoid the disease in this group, and both of them experienced long term effects.
As of today, we have stories from 41 members of MEBO/PATM community and 6 different vaccines: AstraZeneca-Oxford, Johnson & Johnson’s single-shot, Moderna, Pfizer-BioNTech, Sinovac Biotech’s CoronaVac and BBIBP-CorV, also known as the Sinopharm vaccine.
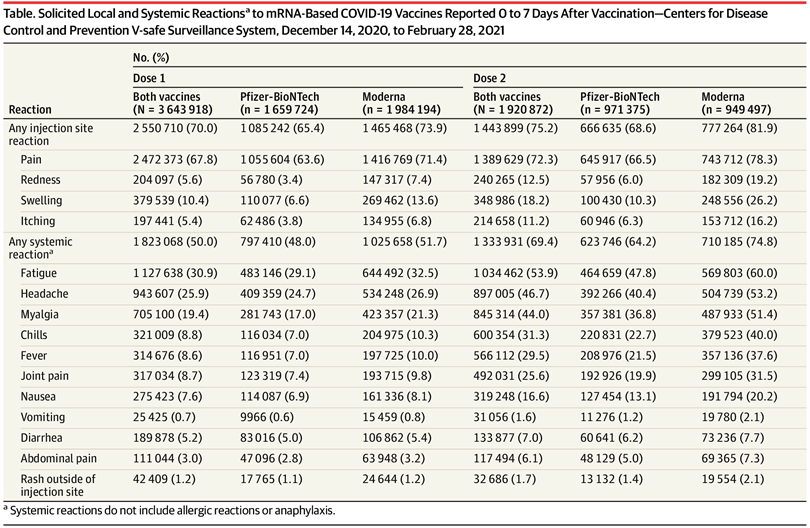
Currently, in various areas of the world, 19 COVID-19 vaccines have been authorized for use. Statistics on short-term effects of these vaccines have been published for different groups. If we compare our data to published data matching by ages and vaccines, short-term effects are very similar. Some of our sub-groups, especially healthy elderly participants, experienced far fewer side effects than reported in the literature. There were slightly fewer common adverse reactions in MEBO Pfizer group, but incidences of fatigue were on a higher side for all vaccines, and there were more reports of fever experienced after Moderna and Astrazeneca, albeit it was not significantly different from the general population. More significant differences were for less common and longer-term effects including fast heartbeat, dry mouth, skin reactions and swollen lymph nodes. The figure below shows common symptoms for Long COVID. Underlined are some of the issues reported after COVID vaccine uptakes in the group. Possible worsening of MEBO/PATM symptoms after vaccinations was reported by 10% of study participants.Why is MEBO/PATM community more susceptible to long COVID? A new study argues that long-haulers might actually be experiencing an attack of fatigue-inducing Epstein-Barr virus (EBV, a member of herpesvirus family HHV-4) that was lying dormant in their bodies. For this study, Gold and his colleagues analyzed blood of 30 people with chronic COVID (out of 185 COVID survivors). 20 out of these 30 carried high levels of EBV antibodies. Vaccines were shown to reactivate viruses too, in much rarer cases. As was demonstrated for Pfizer vaccine that woke up another herpes virus, chickenpox herpes-zoster (HHV-3), that causes shingles when reactivated (this happened to 1% of patients with autoimmune inflammatory rheumatic diseases). Herpes simplex (HSV-1) can be also kept in remission by a healthy immune system and can be also reactivated by COVID-19.
MEBO and PATM symptoms could arise following an infection. Perhaps SARS-CoV-2 can reactivate the old viruses that caused these symptoms to begin with?
Community immunity (also known as herd immunity) protects everyone. We hope that MEBO/PATM community stays COVID-free and safe.