I am always glad to receive letters from people who, despite facing real difficulties, are motivated to understand their condition and actively look for solutions. This letter was one of those.
It came from a young person who had lived with PATM (People Allergic to Me) for just over a year. In that short time, the condition had disrupted education, lab work, friendships, and mental health. Like many others with PATM, this individual had been told - explicitly or implicitly - that what they were experiencing might not be real.
What struck me most was not the suffering (which, sadly, is familiar), but the decision that followed: instead of giving up, they chose to learn, to research, and to ask whether science might eventually provide answers - not only for themselves, but for others.
Below is a modified, bulletized and anonymized version of my response to their questions, shared here because many patients ask the same things.
Why is PATM still an undiagnosed condition?
PATM is often described as “undiagnosed,” but a more accurate term would be not formally recognized.
For a condition to become a recognized clinical entity, several things usually need to be in place:
-
a consistent case definition
-
reproducible, objective measurements
-
and a plausible pathophysiological mechanism that can be validated by multiple independent groups
At present, PATM does not yet meet all of these thresholds.
One major challenge is heterogeneity. The presentation varies widely from person to person, and triggers differ depending on environment, exposure, and individual biology. Another major obstacle is that current clinical workflows are poorly suited to capture intermittent, airborne chemical events. Many patients describe symptoms that occur in bursts—so a clinical visit may appear “normal,” even when the lived experience is not.
What would it take to achieve a formal medical diagnosis?
Large clinical trials can help, but they are rarely the starting point.
The real bottlenecks are:
-
reproducible measurement methods
-
defining subtypes rather than assuming a single mechanism
-
capturing the episodic (“bursty”) nature of emissions
A well-designed, multicenter observational study—with standardized sampling protocols and careful timing relative to symptoms—may be a more realistic bridge step than jumping directly to intervention trials.
An official diagnosis could be beneficial. It can legitimize patients’ experiences in clinical settings, redirect care away from reflexive psychologization and attract more serious research attention. But such a diagnosis has to be built on solid evidence to endure.
Is toluene the main irritation-causing substance?
It is unlikely that there is a single universal compound responsible for PATM.
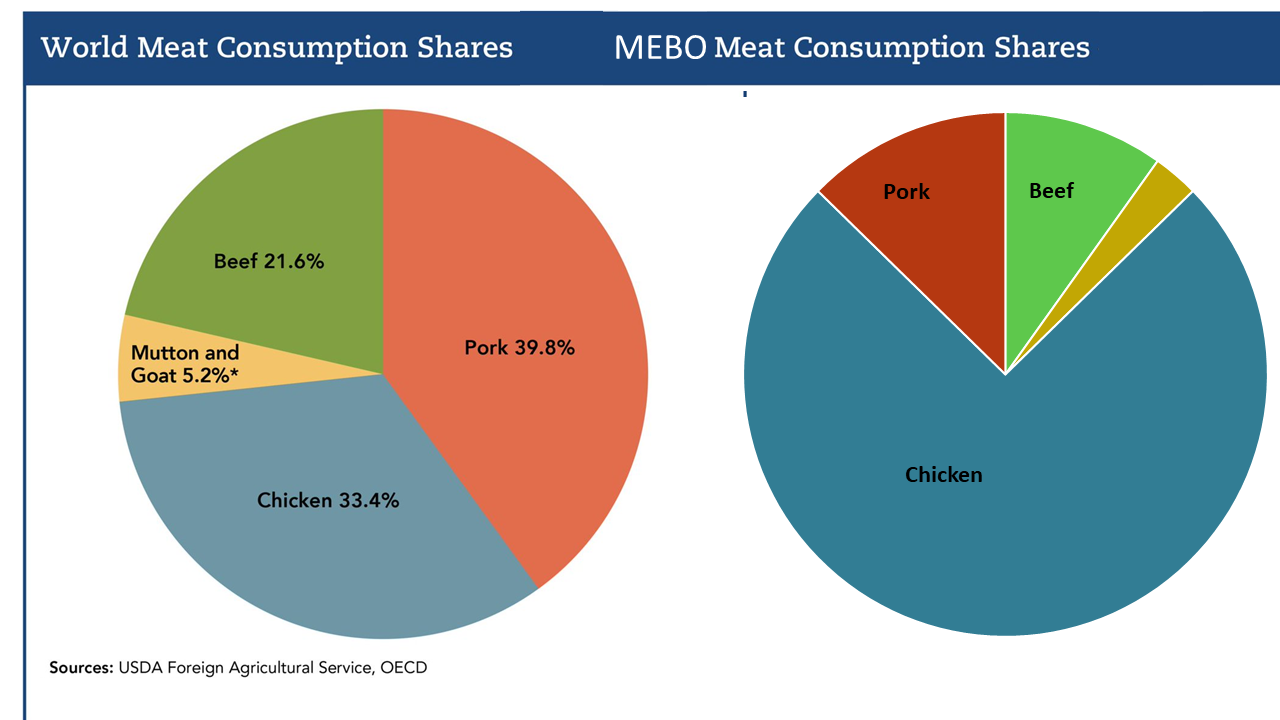
Research on skin gas emission profiles is important because it demonstrates measurable chemical differences, but the broader picture likely involves multiple emitted mixtures and multiple subtypes. In some individuals, compounds such as toluene or related aromatics may contribute to irritation-like symptoms; in others, different chemical patterns may dominate.
Another key factor may be differences in detoxification or clearance. Some people appear more susceptible to everyday exposures—such as secondhand smoke, solvents, or indoor VOCs—not because exposure is higher, but because metabolism and elimination differ.
What can patients realistically try on their own?
I generally recommend starting with low-risk, high-information approaches:
Structured symptom and exposure logging
Tracking timing, diet, stress, environment (workplace, vehicles, indoor air), laundry and personal care products, and proximity to smoke or solvents can help identify repeatable patterns.
Basic medical rule-outs
Even when PATM is the primary concern, it is important to evaluate common contributors to odor or irritation-related conditions, such as reflux, sinus disease, metabolic or endocrine issues, liver and kidney function, medication effects, and dermatologic conditions.
Environmental controls
VOC-related problems are often exposure-amplified. Fragrance-free products, avoiding solvent-heavy cleaners, improving ventilation, and using HEPA plus activated carbon filtration can reduce background “noise” and make patterns easier to recognize.
I generally advise caution with high-risk or expensive interventions unless there is a clear rationale for a particular subtype.
What about fecal microbiota transplants (FMT)?
FMT is scientifically interesting but should be approached with caution. It is not a general solution for PATM and carries nontrivial risks. If considered at all, it should be under appropriate medical supervision and based on a specific, individualized hypothesis—not as a last-resort experiment.
Are microbiome or skin-gas profiling tests useful?
They can be, if used carefully.
-
Gut microbiome profiling may provide clues, but interpretation is still limited and should always be paired with symptom timelines, diet, and repeat measurements.
-
Skin or exhaled gas profiling is conceptually promising because it targets the suspected output directly. However, episodic emissions make timing critical, and passive sampling methods may miss short-lived events.
The usefulness depends less on the technology itself and more on study design.
Would wearable or portable gas sensors help?
In principle, yes. Continuous or frequent measurement could finally correlate chemical signatures with symptoms and environmental context.
In practice, true GC–MS–grade performance in a wearable format remains extremely challenging. Field measurements are complicated by changing ambient air, and episodic emissions require high time resolution and careful baseline correction. The idea is sound; the technology is still catching up.
Could funding agencies support this kind of work?
Possibly more so now than in the past.
Historically, conditions that primarily affect quality of life rather than mortality have struggled to gain funding. When I first applied for support nearly two decades ago, the problem was explicitly described as “not important enough.”
Today, there is broader recognition of the impact of stigma, mental health, and chronic quality-of-life impairment. Advances in exposomics, microbiome science, and wearable sensing technologies make it easier to frame this work as high-risk, high-reward, particularly if the focus is on measurement platforms, subtyping, and mechanism rather than a single compound.
Much of this may sound like a list of obstacles. But compared with even a decade ago, the path forward is clearer.
If PATM turns out not to be one condition but a family of related ones, that is not a failure of science—it is a more accurate description of biology. Progress will likely come not from searching for a single universal cause, but from building frameworks that can accommodate diversity, intermittency, and complexity.
And sometimes, progress begins with a patient who decides that understanding is better than silence.