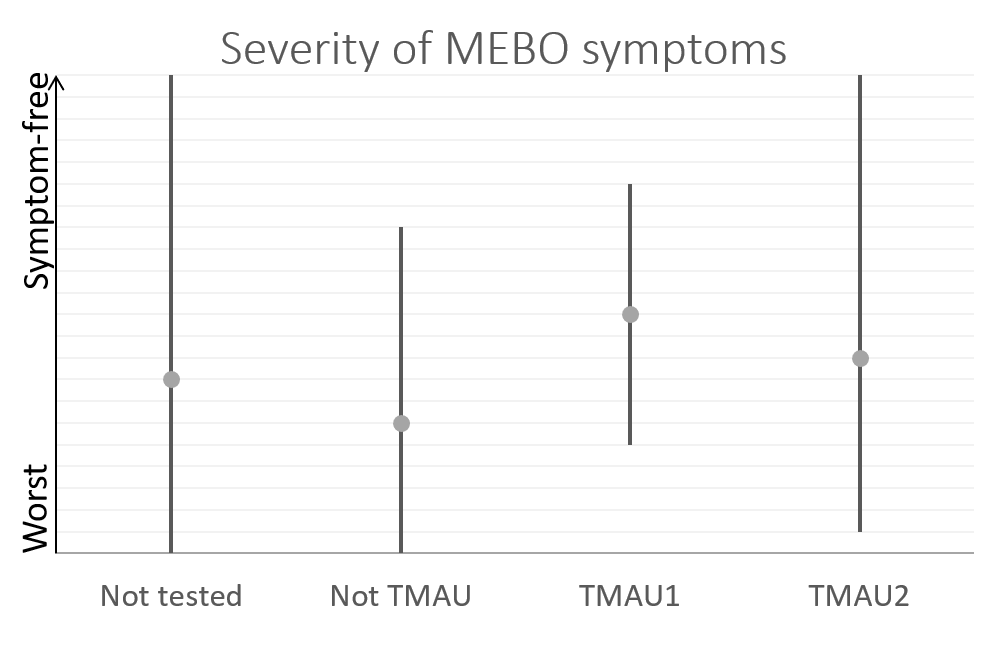
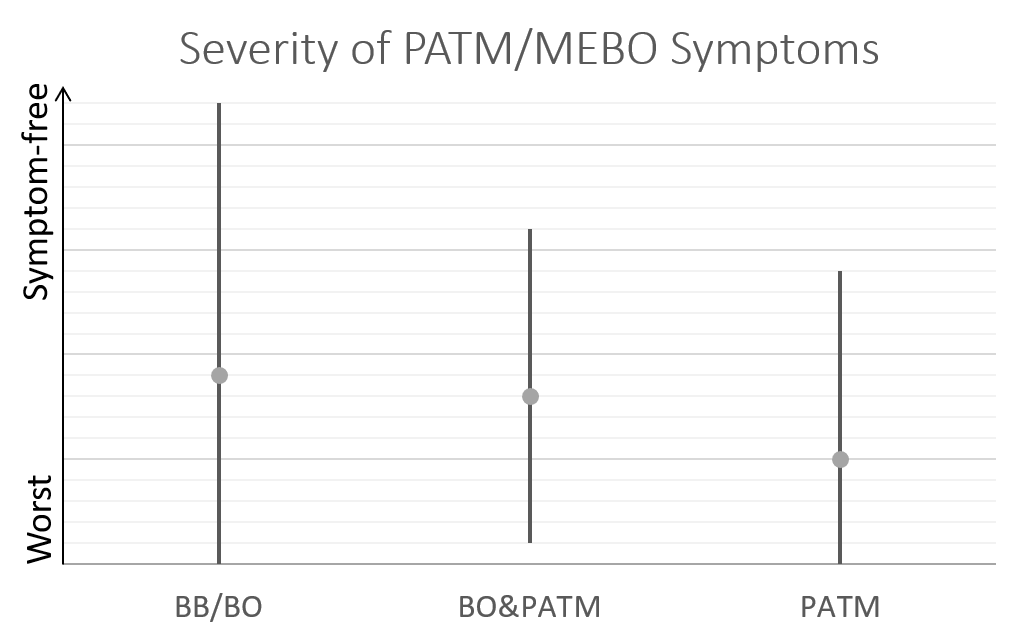
We cannot and do not guarantee or promise that you will receive any benefits from this study.
The alternative is not to participate in this study.
You should not feel obligated to agree to participate. Your questions should be answered clearly and to your satisfaction. If you decide not to participate, tell the Protocol Director.
You will be told of any important new information that is learned during the course of this research study, which might affect your condition or your willingness to continue participation in this study.
A description of this clinical trial will be available on http://www.ClinicalTrials.gov, as required by U.S. Law. This Web site will not include information that can identify you. At most, the Web site will include a summary of the results. You can search this Web site at any time.
The results of this research study may be presented at scientific or medical meetings or published in scientific journals. Your identity and/or your personal health information will not be disclosed except as authorized by you or as required by law. However, there is always some risk that even de-identified information might be re-identified.
Patient information may be provided to Federal and other regulatory agencies as required. The Food and Drug Administration (FDA), for example, may inspect research records and learn your identity if this study falls within its jurisdiction.
Authorization To Use Your Health Information For Research Purposes
Because information about you and your health is personal and private, it generally cannot be used in this research study without your written authorization. If you sign this form, it will provide that authorization. The form is intended to inform you about how your health information will be used or disclosed in the study. Your information will only be used in accordance with this authorization form and the informed consent form and as required or allowed by law. Please read it carefully before signing it.
What is the purpose of this research study and how will my health information be utilized in the study?
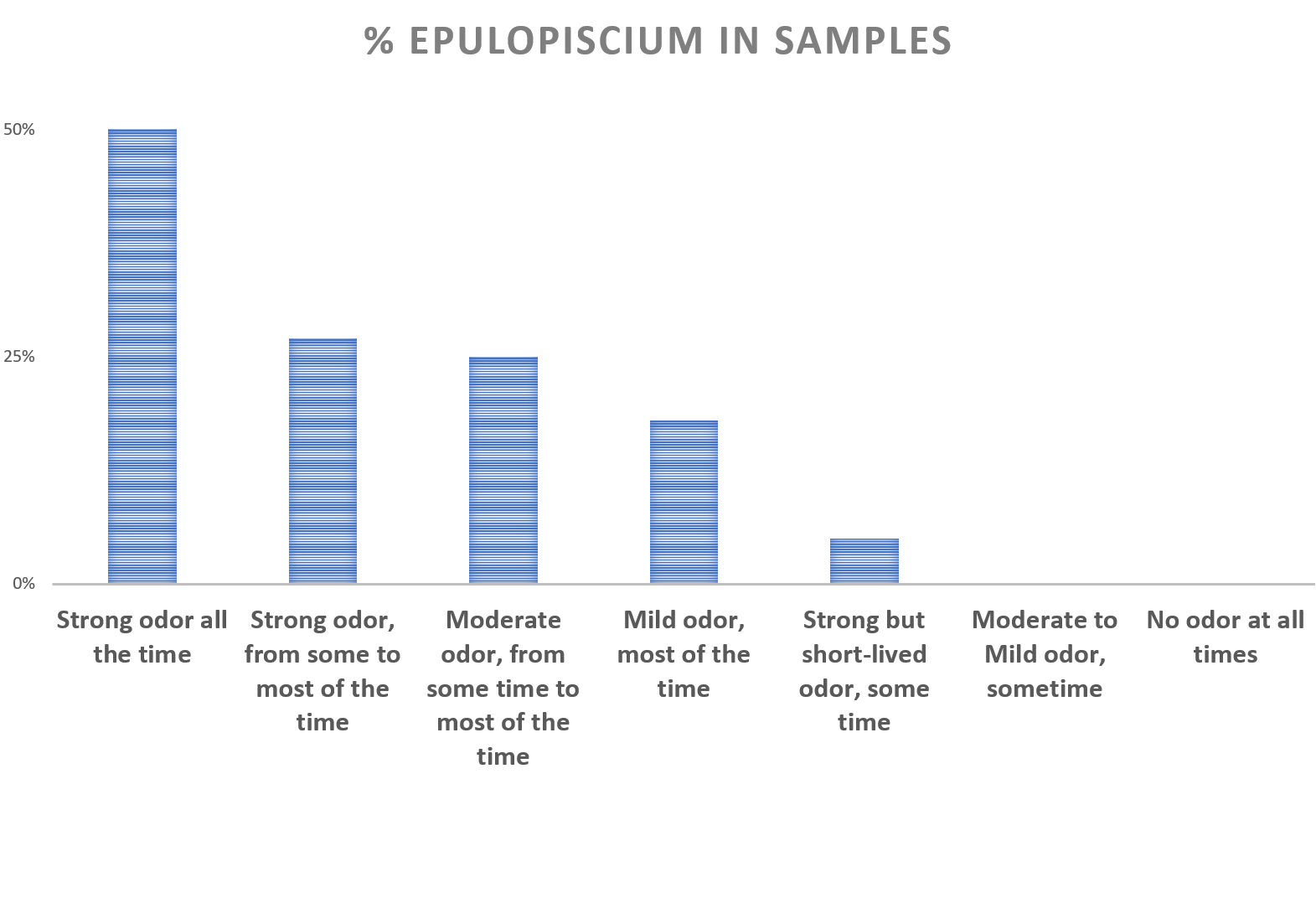
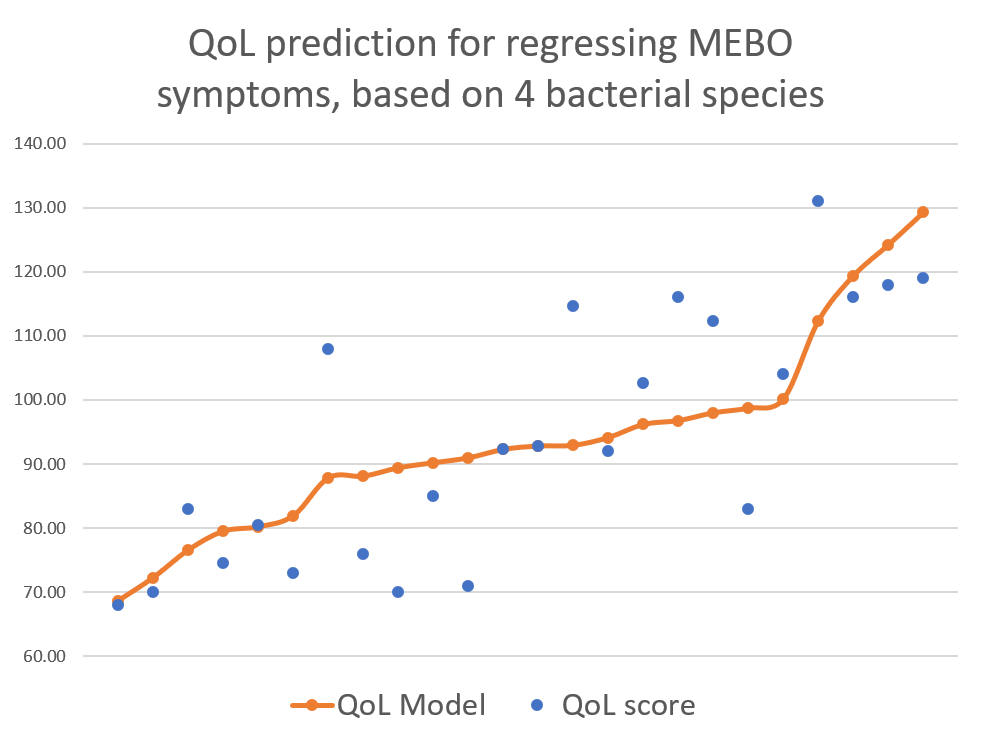
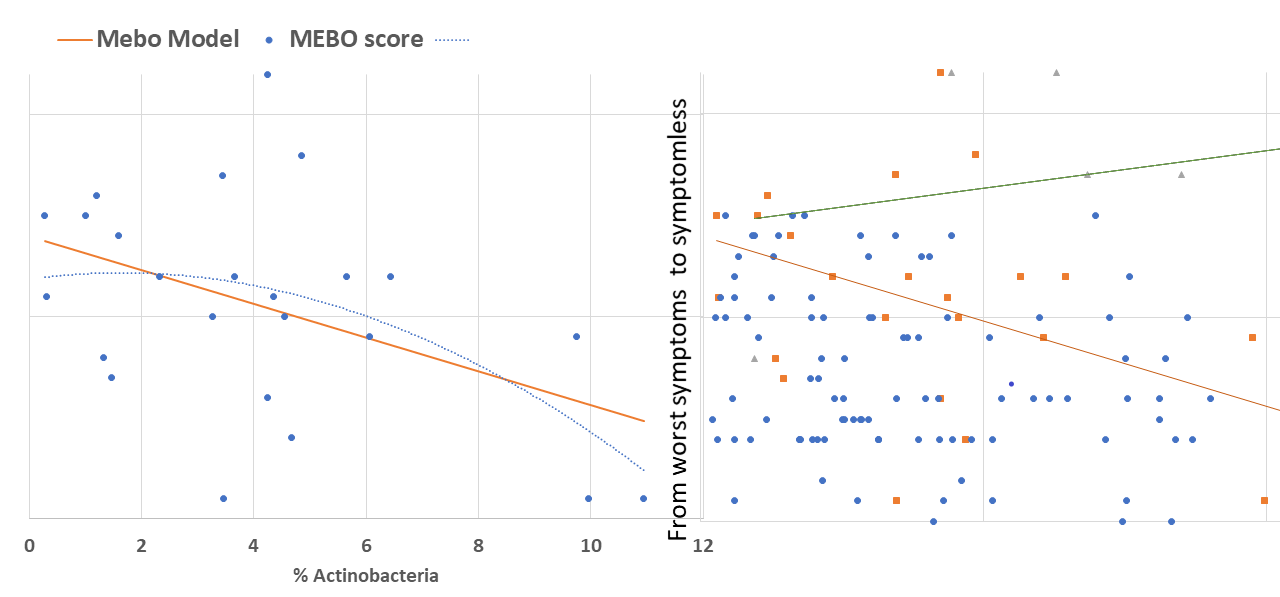
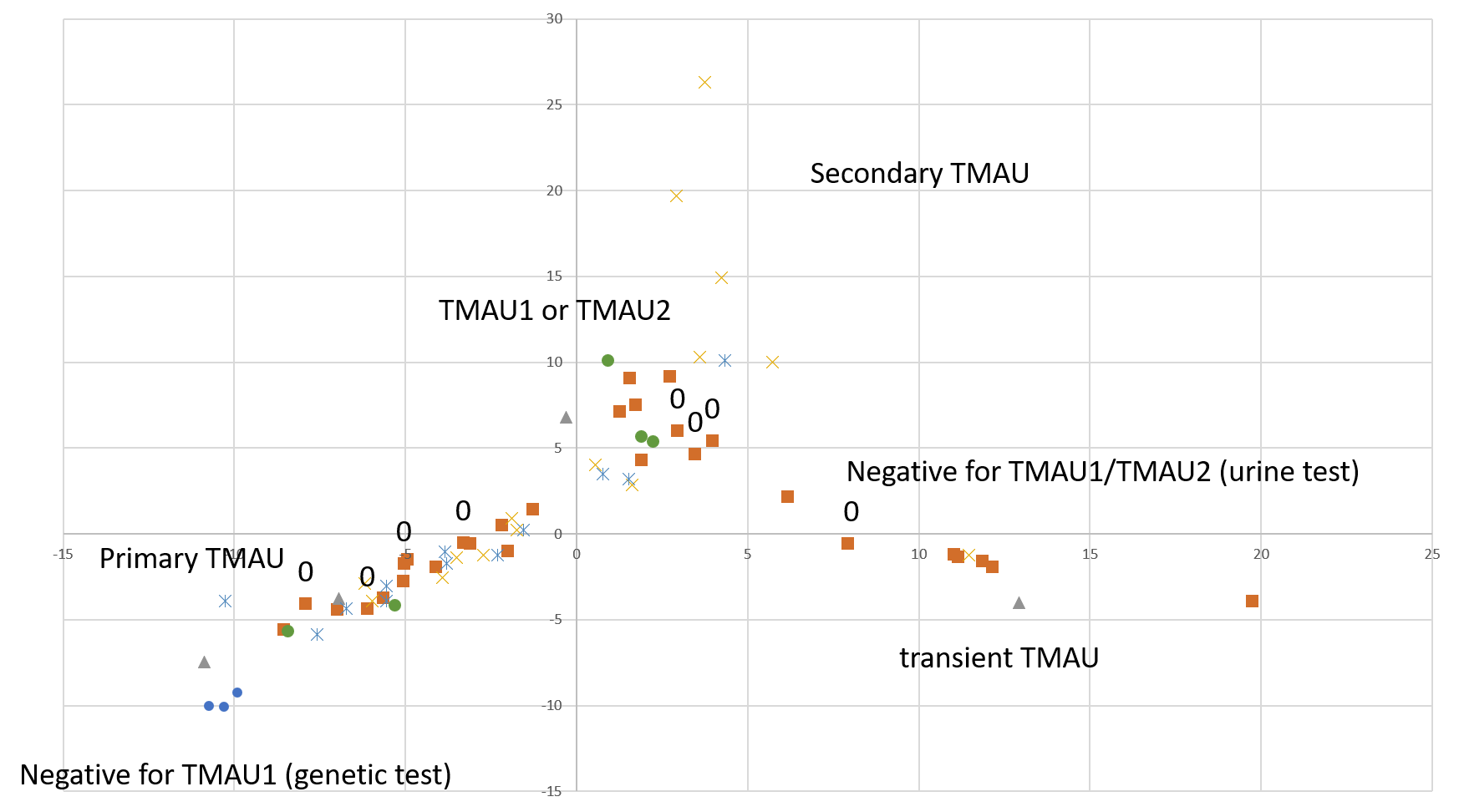
The purpose of this study is to learn what microbial communities are associated with flare ups and remissions of malodor and PATM conditions. The information in some form will be submitted to the sponsor, uBiome. Your de-identified information may be included within pooled summaries when investigators publish results from this study.
Do I have to sign this authorization form?
You do not have to sign this authorization form. But if you do not, you will not be able to participate in this research study. Signing the form is not a condition for receiving any medical care outside the study.
If I sign, can I revoke it or withdraw from the research later?
If you decide to participate, you are free to withdraw your authorization regarding the use and disclosure of your health information (and to discontinue any other participation in the study) at any time. After any revocation, your health information will no longer be used or disclosed in the study, except to the extent that the law allows us to continue using your information (e.g., necessary to maintain integrity of research). If you wish to revoke your authorization for the research use or disclosure of your health information in this study, you must write to Irene Gabashvili at irene.gabashvili@meboresearch.org
What Personal Information Will Be Obtained, Used or Disclosed?
Your health information related to this study, may be used or disclosed in connection with this research study, including, but not limited to severity of your symptoms and laboratory test results.
Who May Use or Disclose the Information?
The following parties are authorized to use and/or disclose your health information in connection with this research study:
· The Protocol Director (Irene Gabashvili)
· Research Staff (Maria de la Torre)
Who May Receive or Use the Information?
The parties listed in the preceding paragraph may disclose your health information to the following persons and organizations for their use in connection with this research study:
· The Office for Human Research Protections in the U.S. Department of Health and Human Services
When will my authorization expire?
Your authorization for the use and/or disclosure of your health information will end on December 31, 2019 or when the research project ends, whichever is earlier.
Will access to my medical record be limited during the study?
To maintain the integrity of this research study, you may not have access to any health information developed as part of this study until it is completed. At that point, you would have access to such health information if it was used to make a medical or billing decision about you (e.g., if included in your official medical record).
Payment/Reimbursement
You will not be paid to participate in this research study.
Costs
There is no cost to you for participating in this study, other than basic expenses like Internet usage and the personal time it will take to fill in the questionnaires.
International participants may be asked to donate to MEBO Research to partially compensate for shipping costs.
Sponsor
uBiome and MEBO Research are providing financial support and/or material for this study. uBiome is supporting microbiome testing and partial analysis of the results, and domestic shipping costs. MEBO Research will be covering International shipping costs.
COMPENSATION for Research-Related Injury
All forms of medical diagnosis and treatment – whether routine or experimental – involve some risk of injury. In spite of all precautions, you might develop medical complications from participating in this study. If such complications arise, the Protocol Director and the research study staff will assist you in obtaining appropriate medical treatment. In the event that you have an injury or illness that is directly caused by your participation in this study, reimbursement for all related costs of care first will be sought from your insurer, managed care plan, or other benefits program. You will be responsible for any associated co-payments or deductibles as required by your insurance.
If costs of care related to such an injury are not covered by your insurer, managed care plan or other benefits program, you may be responsible for these costs. If you are unable to pay for such costs, the Protocol Director will assist you in applying for supplemental benefits and explain how to apply for patient financial assistance from the hospital.
You do not waive any liability rights for personal injury by signing this form.
Questions, Concerns, or Complaints: If you have any questions, concerns or complaints about this research study, its procedures, risks and benefits, or alternative courses of treatment, you should ask the Protocol Director.
Injury Notification: If you feel you have been hurt by being a part of this study, please contact the Protocol Director or Research Staff.
Independent Contact: If you are not satisfied with how this study is being conducted, or if you have any concerns, complaints, or general questions about the research or your rights as a participant, please contact the MEBO Institutional Review Board (IRB) to speak to someone independent of the research team at mike@meboresearch.org
Alternate Contact: If you cannot reach the Protocol Director, please contact Maria de la Torre at maria.delatorre@meboresearch.org
Questions, Concerns, or Complaints: If you have any questions, concerns or complaints about this research study, its procedures, risks and benefits, or alternative courses of treatment, you should ask the Protocol Director, Irene Gabashvili at irene.gabashvili@meboresearch.org. You should also contact her at any time if you feel you have been hurt by being a part of this study.
EXPERIMENTAL SUBJECT’S BILL OF RIGHTS
As a research participant you have the following rights. These rights include but are not limited to the participant's right to:
- be informed of the nature and purpose of the experiment;
- be given an explanation of the procedures to be followed in the medical experiment, and any drug or device to be utilized;
- be given a description of any attendant discomforts and risks reasonably to be expected;
- be given an explanation of any benefits to the subject reasonably to be expected, if applicable;
- be given a disclosure of any appropriate alternatives, drugs or devices that might be advantageous to the subject, their relative risks and benefits;
- be informed of the avenues of medical treatment, if any available to the subject after the experiment if complications should arise;
- be given an opportunity to ask questions concerning the experiment or the procedures involved;
- be instructed that consent to participate in the medical experiment may be withdrawn at any time and the subject may discontinue participation without prejudice;
- be given a copy of the signed and dated consent form; and
- be given the opportunity to decide to consent or not to consent to a medical experiment without the intervention of any element of force, fraud, deceit, duress, coercion or undue influence on the subject's decision.